Abstract
Background: Genomic heterogeneity in leukemic blasts characterizes Acute Myeloid Leukemia (AML) patients and is associated to variable drug response. However, use of genomics to guide therapy has generally been restricted to a single-gene approach, which rarely has sufficient predictive power to be clinically useful. Comprehensive DNA sequencing and biosimulation of the Computational Omics Biology Model (CBM) provide the opportunity and means of predicting treatment outcome in advance of treatment.
Methods: The Cellworks CBM is a computational multi-omic biology software model created using artificial intelligence heuristics and literature sourced from PubMed, to generate a patient-specific protein network map. The CBM permits mapping of biological pathways associated with tumorigenesis and drug resistance using mathematical principles to yield a virtual tumor model that can be used in biosimulation. Aberration and copy number variations from each case served as input to the CBM to generate individual patient-specific protein network maps. We used the Cellworks Biosimulation Platform to identify novel genomic biomarkers associated with response among AML patients treated with Cytarabine (ARA-C) + idarubicin or daunorubicin (anthracycline) with or without Etoposide (VP16).
539 AML patients were selected for this study based largely on genomic data published in TCGA and PubMed:
ARA-C + daunorubicin [N=111, 92 responders (R) & 19 non-responders (NR)]
ARA-C + idarubicin [N=109, 94 R & 15 NR]
ARA-C + daunorubicin + VP16 [N=6, 4 R & 2 NR]
ARA-C + idarubicin + VP16 [N=313, 261 R & 52 NR]
Drug impact on individual disease networks was simulated to determine efficacy value by measuring the effect of chemotherapy on the cell growth score, a composite of cell proliferation, viability, apoptosis, metastasis, DNA damage and other cancer hallmarks. The mechanism of action of each drug was used to map its biological consequences to each patient's cancer genome to predict treatment response.
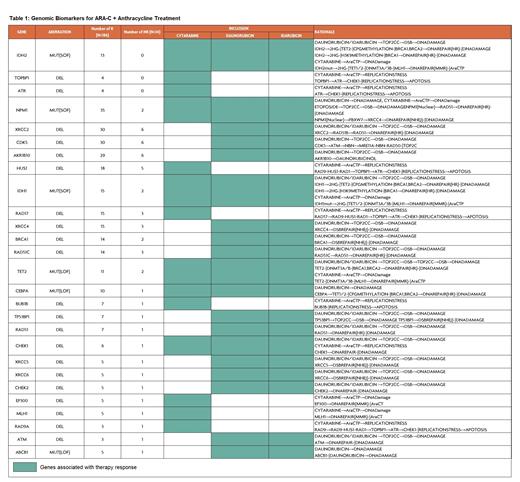
Results: Biosimulation of ARA-C + anthracycline with and without VP16 identified biomarkers responsible for therapy response. Additionally, the Cellworks Biosimulation Platform identified novel drug combinations for NR to these standard combinations. There were 186/220 patients treated with ARA-C + anthracycline that had clinical responses. Major biomarkers predictive of response included: IDH2 mut, TOPBP1 del, ATR del, NPM1 mut, IDH1 mut, XRCC2 del, CDK5 del, AKR1B1 del and other genes (Table 1). The frequency of these genes was significantly higher (exact binomial: p-value < 0.0001) in R (N=186) vs. NR (N=34). Notably, 7/34 NR to the two-drug combination had favorable biomarkers for VP16 response, which included RAD52 del, FANCD2 del, STAG2 mut, MPO amp, and NHEJ del. On the other hand, among 265 R treated with triplet therapy (ARA-C + anthracycline + VP16), 30 patients were unlikely to have derived incremental benefit from the addition of VP16. In these patients, the biosimulation predicted that they would have benefited equally from doublet therapy (ARA-C + anthracycline without VP16). In this subgroup of R, ARID1A del, FLT3-ITD mut, GSTA1 amp, KEAP1 del, or RNF1 del generated resistance to VP16 in the biosimulation. Among 54 NR to triplet therapy, 40/54 had genomic alterations predicting a benefit from JQ1, BRD2/4 inhibitors, including KMT2C del, FLT3 GOF, NPM1 del, DNMT3A LOF, and TP53 del, while 12 patients had 5q del highlighting a potential benefit from lenalidomide. Altogether, 89/539 (16.5%) could have been managed with a potentially superior treatment approach based on the biosimulation by either adding or omitting VP16 or being treated with an alternative therapy.
Conclusions: Cellworks Biosimulation Platform applied to the patient-specific CBM identifies novel biomarkers of response and can be employed to determine the optimal therapy for AML patients. This study highlights patients for whom triplet therapy promises potentially superior benefit, others who would benefit equally from doublet therapy without VP16, and others unlikely to respond to standard or triplet therapy for whom an alternative personalized approach might offer better outcomes. In AML, biosimulation offers the possibility to tailor the chemotherapy regimen to each patient to improve disease control and minimize toxicity.
Castro: Caris Life Sciences Inc.: Consultancy; Guardant Health Inc.: Speakers Bureau; Bugworks: Consultancy; Cellworks Group Inc.: Current Employment; Omicure Inc: Consultancy; Exact sciences Inc.: Consultancy. Howard: Servier: Consultancy; Cellworks Group Inc.: Consultancy; Sanofi: Consultancy, Other: Speaker fees. Kumar: Cellworks Group Inc.: Current Employment. Patil: Cellworks Group Inc.: Current Employment. Khandelwal: Cellworks Group Inc.: Current Employment. Watson: BioAi Health: Consultancy, Membership on an entity's Board of Directors or advisory committees; AlloVir: Consultancy, Membership on an entity's Board of Directors or advisory committees; CellMax Life: Consultancy, Other: Advisor; Cellworks Group Inc.: Consultancy, Other: Advisor. Kapoor: Cellworks Group Inc.: Current Employment. Kumari: Cellworks Group Inc.: Current Employment. Prasad: Cellworks Group Inc.: Current Employment. Gupta: Cellworks Group Inc.: Current Employment. Lunkad: Cellworks Group Inc.: Current Employment. Mitra: Cellworks Group Inc.: Current Employment. G: Cellworks Group Inc.: Current Employment. Kumar: Cellworks Group Inc.: Current Employment. Choudhury: Cellworks Group Inc.: Current Employment. Kulkarni: Cellworks Group Inc.: Current Employment. Choudhary: Cellworks Group Inc.: Current Employment. Prakash: Cellworks Group Inc.: Current Employment. Husain: Cellworks Group Inc.: Current Employment. Ghosh: Cellworks Group Inc.: Current Employment. Narvekar: Cellworks Group Inc.: Current Employment. Amara: Cellworks Group Inc.: Current Employment. Yuvavani: Cellworks Group Inc.: Current Employment. Patel: Cellworks Group Inc.: Current Employment. Macpherson: Cellworks Group Inc.: Current Employment. Marcucci: Novartis: Other: Speaker and advisory scientific board meetings; Abbvie: Other: Speaker and advisory scientific board meetings; Agios: Other: Speaker and advisory scientific board meetings.